Research
The brain can flexibly adapt the physiology and behaviors of the body to specific life-stage demands. For example, sexual maturation is initiated when the maturation and nutritional state of the body are ready for reproduction. Parental caregiving behaviors to infants are facilitated around the time when their own young are expected. Specific neural activity patterns that regulate milk ejection only emerge in lactating mothers. These examples suggest the presence of neural mechanisms that plastically adjust neural input organizations and output functions based on an internal state of organisms. However, little is known as to how such adaptive plasticity is implemented at the level of neural circuits. Our research aims to elucidate the mechanisms of flexible neuronal functions associated with different life stages in mice by targeting a diverse array of brain regions, ranging from the frontal cortex to the sympathetic nervous system. We employ a combination of cutting-edge techniques, including single-cell RNAseq, virus-based circuit mapping, in vivo imaging of neural activity, and molecular and neural manipulation of specific neuron types. Through our work, we hope to provide novel insights into the plasticity of the nervous system that underlies the control of animal behavior and body physiology.

Herein, we delineate the current research themes that our laboratory is actively pursuing. Nevertheless, we remain receptive to exploring other themes that delve into the mechanisms of brain plasticity across diverse life stages and conditions. Furthermore, we endeavor to push the boundaries of technological development, which serve to enhance our understanding of these mechanisms on a broader scope. Should you be interested in our laboratory, please feel free to reach out to the Principal Investigator at kazunari.miyamichi [at] riken.jp.
- 1. A Neural Basis of Malnutrition-mediated Pubertal Failure in Female Mice
Reproduction imposes a heavy burden, particularly on mammalian females. Consequently, the acquisition of reproductive capacity is tightly regulated by the body's energy status. In both in women and rodent models, chronic energy deficiency, such as that induced by malnutrition, is associated with delayed puberty. The onset of puberty is governed by the pulsatile secretion of gonadotropin-releasing hormone (GnRH), the master regulator of gonadal functions. GnRH neurons are primarily controlled by kisspeptin neurons in the hypothalamic arcuate nucleus (ARCkiss), the pulse generator of GnRH. We found that the pulsatile activity of ARCkiss neurons declines in response to food restriction but recovers markedly within hours of restored food availability. We also identified agouti-related peptide (Agrp) neurons in the arcuate nucleus (ARCAgrp)—a key hub for hunger sensing—as regulators of ARCkiss neuron pulsatile activity, playing a pivotal role in coordinating the timing of sexual maturation. These findings reveal a mechanism by which reproductive centers dynamically adjust to changes in dietary availability, offering new insights into the regulation of sexual and reproductive health.
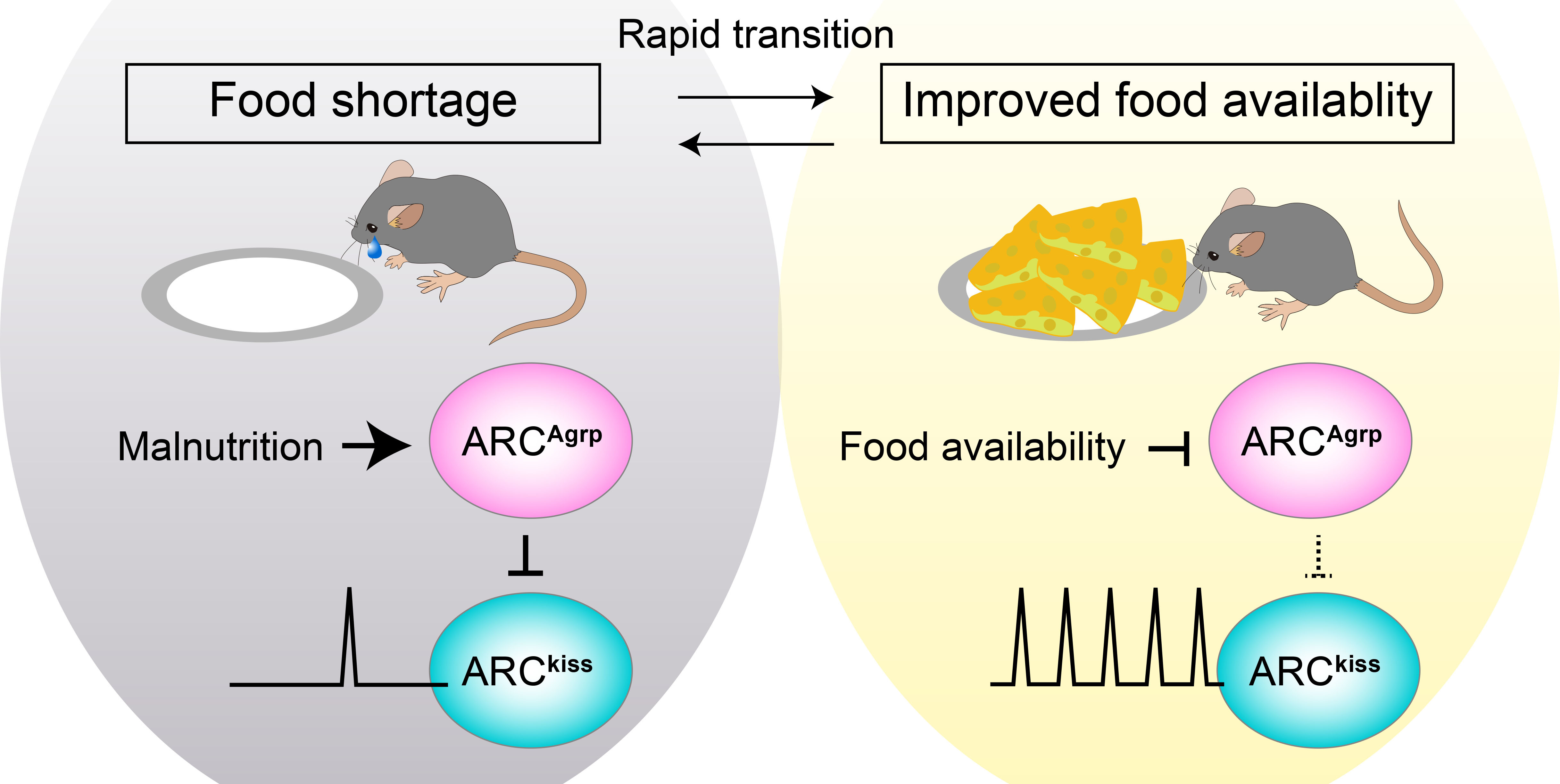
Dietary availability acutely influences puberty onset via a hypothalamic neural circuit.
Goto T#, Hagihara M, Irie S, Abe T, Kiyonari H, Miyamichi K#. Neuron (2025)
DOI: https://doi.org/10.1016/j.neuron.2025.01.015
- 2. Regulation of Paternal Caregiving Behaviors
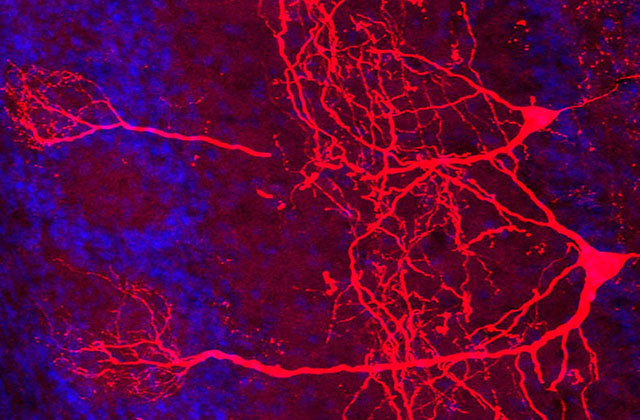
Male mice, before mating, exhibit aggressive behavior towards young; however, upon mating and becoming fathers, they manifest nurturing (caregiving) behaviors such as keeping the offspring warm and retrieving the offspring that have scattered from the nest. Our research focuses on investigating the role of oxytocin neurons in the paraventricular nucleus of the hypothalamus in the manifestation of these caregiving behaviors. Our findings indicate that inhibiting oxytocin synthesis in these neurons leads to neglect of the pups by the paternal mice. Conversely, activating the oxytocin neurons elicits caregiving behavior even in unmated males, suggesting that the oxytocin neurons are crucial in regulating paternal caregiving behavior. Furthermore, we discovered that paternal mice undergo changes in neural input that facilitate the activation of the oxytocin neurons, thus contributing to the emergence of caregiving behaviors.

Plasticity of Neural Connections Underlying Oxytocin-mediated Parental Behaviors of Male Mice.
Inada K, Hagihara M, Tsujimoto K, Abe T, Konno A, Hirai H, Kiyonari H, Miyamichi K. Neuron, (2022) doi: 10.1016/j.neuron.2022.03.033
See also wonderful preview by Dr. Kim: Fatherhood is life-changing: Uncovering structural and functional changes in the dad brain - PubMed (nih.gov)
Next, we examined vasopressin, a related hormone produced in the paraventricular nucleus of the hypothalamus. Ablating vasopressin-producing neurons suppressed paternal behavior in male mice, whereas activating these neurons enhanced it. Moreover, deleting oxytocin receptors in the preoptic area abolished the ability of vasopressin to promote paternal behavior. These findings indicate that vasopressin released from the paraventricular nucleus facilitates paternal care by engaging crosstalk to oxytocin receptor in the preoptic area.

Vasopressin-to-oxytocin receptor crosstalk in the preoptic area underlying parental behaviors in male mice
,Hagihara M*, Yaguchi K, Irie S, Inoue YU, Inoue T, and .
Nature Communications 16, 10844, 2025.
DOI: 10.1038/s41467-025-66908-0
- 2’ Satiety Formation and the Role of Oxytocin
While appetite is a fundamental need for animals, the brain also possesses the ability to prevent excessive food intake. When mice consume an appropriate amount of food, they halt further feeding, likely due to the activation of neural circuits that suppress appetite in the brain. Although the involvement of oxytocin in the control of feeding has been postulated, the precise mechanisms have remained unclear. In our research, we discovered that mice with a conditional knockout of the oxytocin gene in the paraventricular nucleus of the hypothalamus exhibited hyperphagic obesity. Furthermore, similar results were observed when oxytocin receptors were conditionally deleted in the arcuate nucleus. These findings demonstrate the existence of an oxytocin-mediated neural circuit that suppresses appetite and thus provides insights into the neural underpinnings of appetite control.

Oxytocin signaling in the posterior hypothalamus prevents hyperphagic obesity in mice.
Inada K, Tsujimoto K, Yoshida M, Nishimori K, Miyamichi K. eLife, (2022) doi: 10.7554/eLife.75718
- 3. Neuroscience of Parturition and Lactation
Oxytocin, a hormone that plays a crucial role in maternal physiology, exerts its effects on uterine contractions during labor and is often administered as a labor-inducing agent. In addition, oxytocin is necessary for the process of lactation, whereby milk is released from the mammary glands. Oxytocin secretion occurs in waves or pulses from the pituitary gland, with each wave reaching the uterus and mammary glands every few minutes. However, the neural circuit and molecular mechanisms that govern this rhythmic activity are not yet fully understood. Using advanced genetic tools in mice, we have been able to precisely record and analyze the activity of oxytocin neurons during parturition and lactation. Through our research, we have demonstrated that the pulsation of oxytocin in the mother can be artificially manipulated by selectively activating specific neurons based on mapping the neural inputs to oxytocin neurons.

Recording and manipulation of the maternal oxytocin neural activities in mice.
Yukinaga H, Hagihara M, Tsujimoto K, Chiang H-L, Kato S, Kobayashi K, Miyamichi K. Current Biology, (2022) doi: 10.1016/j.cub.2022.06.083
Based on this work, we are currently exploring the neural circuitry mechanisms that govern the generation of pulsatile activities of oxytocin neurons in the mother through the manipulation of upstream neural circuitry and gene functions. As a significant step towards facilitating this investigation, we have developed an uncomplicated method that does not require the use of Cre recombinase, which enables the efficient capture of oxytocin neuron pulsatile activity in wild-type animals and various genetically modified mice.
Dynamic modulation of pulsatile activities of oxytocin neurons in lactating wild-type mice.
Yaguchi K, Hagihara M, Konno A, Hirai H, Yukinaga H, Miyamichi K. PLoS One, (2023) doi: 10.1371/journal.pone.0285589
Studies on systemic (whole-body) oxytocin knockout mice have indicated that oxytocin may not be crucial for partition or caregiving behavior in mother mice. We have re-evaluated these findings with oxytocin conditional knockout mice. Our results suggest that oxytocin gene deletion from both the paraventricular (PVH) and supraoptic (SON) nucleus does not result in a significant phenotype in the partition or caregiving behavior of mother mice, supporting the classical studies. Interestingly, our examination of the classical function of oxytocin - lactation - revealed that deletion of the oxytocin gene from the PVH did not produce any significant defects, whereas severe lactation defects appeared when the number of oxytocin neurons in the SON fell below 200. These results indicate an essential role of oxytocin derived from SON for milk ejection, which may offer valuable insights into exploring the neural mechanisms of lactation.
The importance of oxytocin neurons in the supraoptic nucleus for breastfeeding in mice.
Hagihara M, Miyamichi K, Inada K. PLoS One, (2023) doi: 10.1371/journal.pone.0283152.
From the above study, it was known that pulsatile activity of oxytocin neurons becomes larger and longer during the mid-lactation period compared to early lactation. However, changes in the activity of individual neurons remained unclear. To address this, we established a single-cell imaging technique using microendoscopy. We show that during lactation, many oxytocin neurons synchronously participate in pulsatile activity. In mid-lactation, the number of active neurons increases, and the pulse amplitude of individual neurons also expands, resulting in an overall enhancement of neuronal activity. Conversely, in post-weaning dams, despite the inability to produce milk, pulsatile activity persists at a lower level. This is characterized by a reduction in the number of active neurons and narrower pulse amplitudes, indicating a return to a basal state. These changes suggest a mechanism that dynamically increases oxytocin secretion during periods of heightened demand for maternal milk, with individual oxytocin neurons being flexibly adjusted. This study opens a new avenue of neuroendocrinology by enabling real-time, single-cell-level observations in freely behaving animals.

Flexible Adjustment of Oxytocin Neuron Activity in Mouse Dams Revealed by Microendoscopy.
Yaguchi K, Miyamichi K, and Tasaka G. Science Advances, (2024) Doi: 10.1126/sciadv.adt1555
- 4. A Prefrontal Neural Circuit for Maternal Behavioural Learning in Mice
Maternal caregiving behavior, essential for the survival of mammalian offspring, is often regarded as instinctive. However, it also includes components that are initiated and refined through experience and learning. How the brain efficiently acquires such evolutionarily vital behaviors remains poorly understood at the level of neural circuits. Prevailing circuit models of parental care propose that the prefrontal cortical network integrates sensory cues from the young and governs decision-making and motivational processes. Yet, these hypotheses have lacked functional validation, and the precise role of the prefrontal cortex in caregiving behavior remains unclear. Here we find that when virgin female mice are co-housed with lactating dams, the orbitofrontal cortex (OFC) facilitates the efficient acquisition of alloparental caregiving behaviors. Using microendoscopic calcium imaging in freely moving mice, we identified a substantial population of OFC neurons that are activated during pup retrieval. Notably, these neural representations significantly overlapped with those associated with reward responses to sucrose. Furthermore, opto- and chemogenetic suppression of the OFC during early stages of caregiving learning led to reduced activity in ventral tegmental area (VTA) dopamine neurons and declined dopamine release in the nucleus accumbens. These findings suggest that OFC-mediated enhancement of dopaminergic signaling supports the effective learning of maternal caregiving behavior in mice.
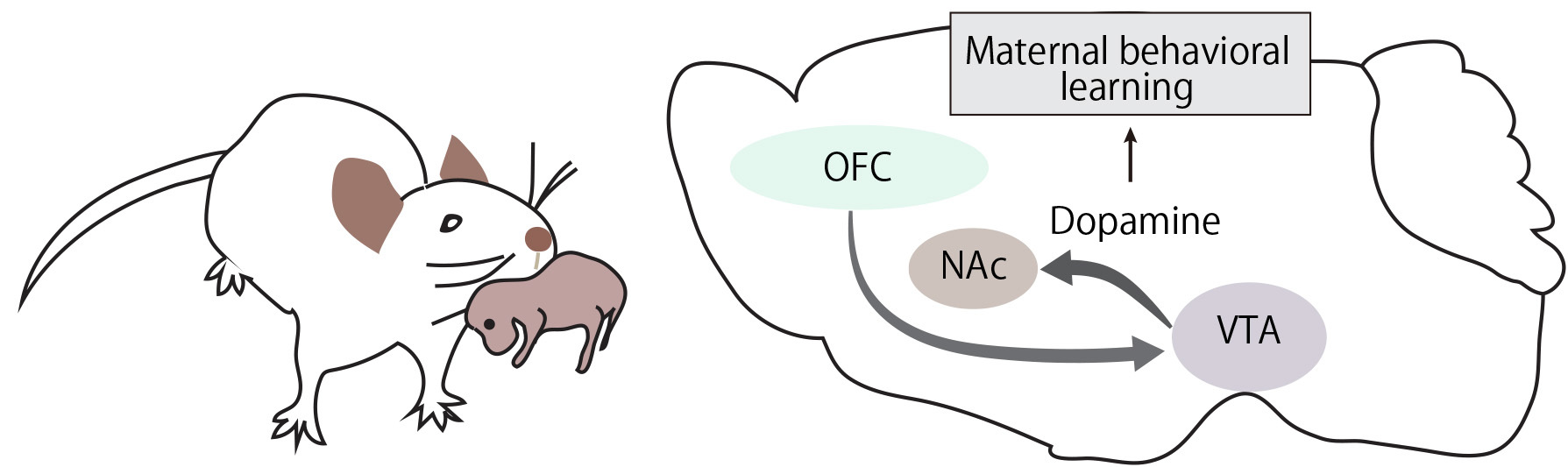
Orbitofrontal cortex influences dopamine dynamics associated with alloparental behavioral acquisition in female mice.
Tasaka G, Hagihara M, Irie S, Kobayashi H, Inada K, Kobayashi K, Kato S, Kobayashi K, and Miyamichi K.
Science Advances, (2025) doi: 10.1126/sciadv.adr4620
- 5. A Neural Activity during Reproductive Senescence
The transition to reproductive senescence significantly impacts the quality of life of women, but the underlying neural mechanisms remain poorly characterized. We establish long-term (from the reproductive to acyclic phase) chronic imaging of the central pacemaker activities of reproductive functions by fiber photometry in female mice. In particular, we focus on kisspeptin neurons in the arcuate nucleus (ARCkiss) of the hypothalamus. Our data reveal that during the transition to reproductive senescence, the pulsatile activities of ARCkiss show unexpected robustness in terms of frequency, but a tendency for the intensity to decline. Their findings exhibit the power of direct chronic visualization of hormonal regulators in the brain, which is generally applicable to facilitate studies of aging-associated malfunctions in neuroendocrine systems.
Dynamics of pulsatile activities of arcuate kisspeptin neurons in aging female mice.
Goto T, Hagihara M, Miyamichi K. eLife, (2023) doi: 10.7554/eLife.82533
- 6. Neural Basis of Social Dysfunctions Developed by Environmental Factors
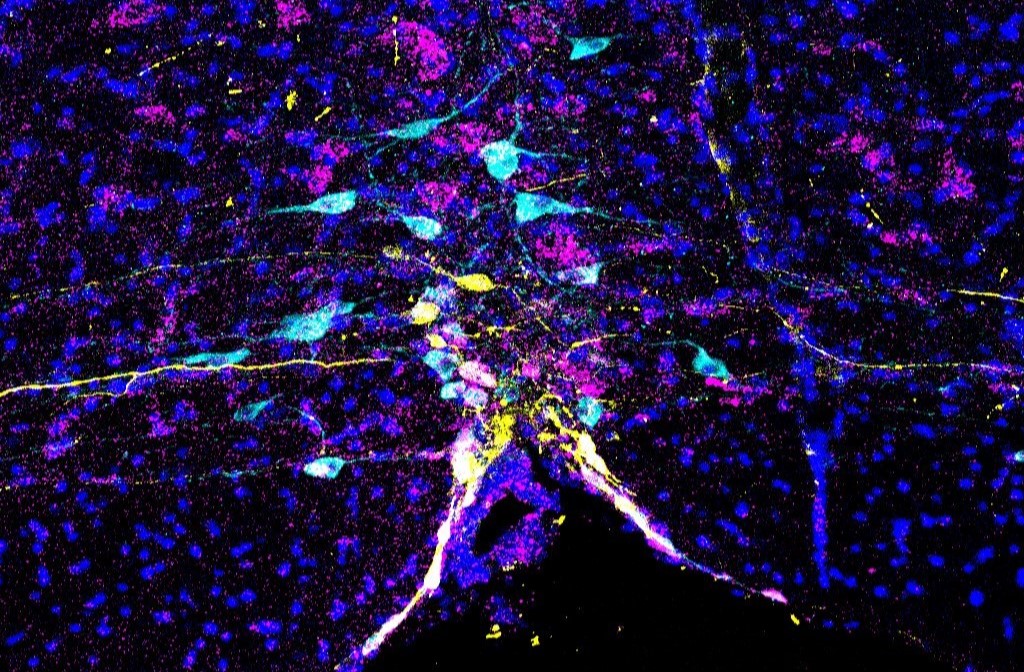
Selective vulnerability offers a conceptual framework for understanding neurodegenerative disorders such as Parkinson’s disease, where specific neuronal types are selectively affected and adjacent ones are spared. However, the applicability of this framework to neurodevelopmental disorders, particularly those characterized by atypical social behaviors, such as autism spectrum disorder, remains uncertain. We show that an embryonic disturbance, known to induce social dysfunction in male mice, preferentially impaired the gene expression crucial for neural functions in parvocellular oxytocin (OT) neurons—a subtype linked to social rewards—while neighboring cell types experienced a lesser impact. Chemogenetic stimulation of OT neurons at the neonatal stage ameliorated social deficits in early adulthood, concurrent with cell-type-specific sustained recovery of pivotal gene expression within parvocellular OT neurons. Our data shed light on the transcriptomic selective vulnerability within the hypothalamic social behavioral center and provide a potential therapeutic target through specific neonatal neurostimulation.
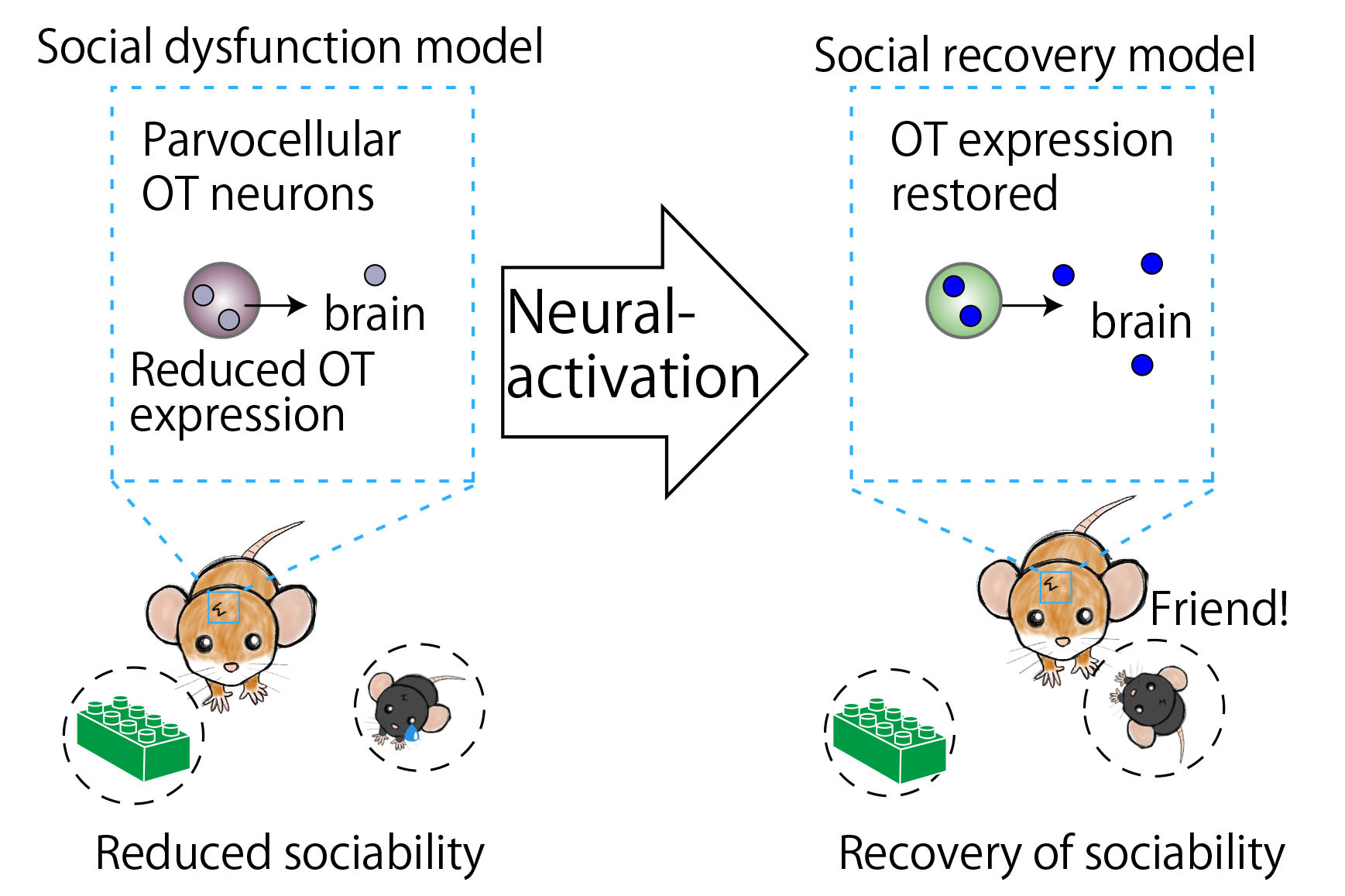
Selective Vulnerability of Parvocellular Oxytocin Neurons in Social Dysfunction.
Tsurutani M, Goto T, Hagihara M, Irie S, Miyamichi K. Nat Commun. 15: 8661 (2024), doi: 10.1038/s41467-024-53092-w.
- 7. Sympathetic Nervous Efferents in the Downstream of the Hypothalamus
The hypothalamus orchestrates a wide range of physiological functions and maintains homeostasis through two principal output systems: the neuroendocrine and autonomic nervous systems. The autonomic nervous system operates involuntarily to regulate critical organs, including the circulatory, respiratory, digestive, and reproductive systems, and plays a pivotal role in adapting to environmental challenges. While the neuroendocrine system provides uniform, body-wide signals, the autonomic nervous system offers the advantage of delivering precise, organ-specific adjustments. However, a fundamental question remains: does the brain indeed regulate each organ individually, and what neural architecture underpins this specificity? Our recent studies have addressed this question by examining sympathetic preganglionic neurons in the lower thoracic spinal cord, focusing on their regulation of the adrenal gland (an endocrine organ) and intestinal motility. We identified molecularly distinct subtypes of sympathetic neurons that independently control these functions. Moreover, under conditions of carbohydrate deprivation, the adrenal pathway, which enhances blood glucose levels, was selectively activated, whereas the intestinal pathway exhibited minimal activation. These results highlight the existence of specialized, molecularly defined sympathetic circuits dedicated to specific organ groups. Ongoing investigations seek to establish whether this organizational principle extends to the regulation of other organ systems.

Parallel Labeled-Line Organization of Sympathetic Outflow for Selective Organ Regulation in Mice.
Harima Y, Tsurutani M, Yamada S, Uchida S, Inada K, Hagihara M, Irie S, Shigeta M, Abe T, Inoue YU, Inoue T, Miyamichi K. Nature Communications, (2024) DOI: 10.1038/s41467-024-54928-1